Clinical SNP association and GWAS
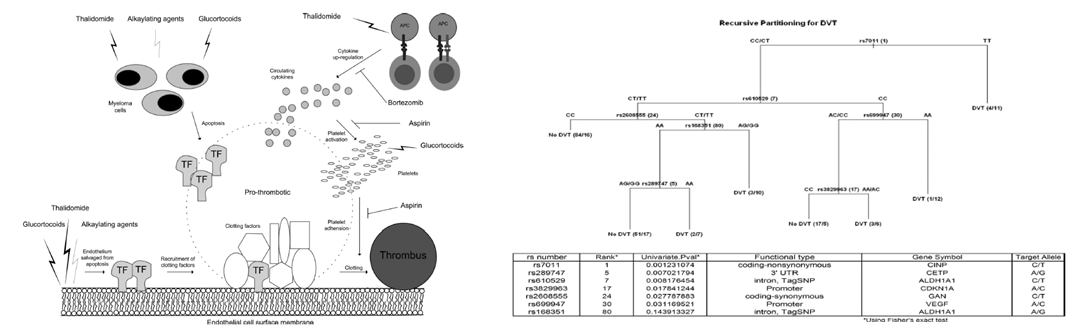
Thalidomide related venous thrombosis
Taking a gene list developed together with US and Dutch collaborators, I designed a custom SNP array of 3,400 SNP from an early dbSNP and a number of pathway-oriented SNP databases. The
array focused on non-synonymous variants and SNP with described functionality. We performed a SNP association study with venous thrombosis in a case-control analysis of thalidomide patients,
combining the results from three clinical trials. Careful reexamination of the serious adverse reports was required to ensure the appropriate distinction between venous thrombosis and
atrial thrombosis which have different mechanisms, with atrial events not symptomatic of thalidomide-related toxicity. Results from the thalidomide-related analysis was contrasted with
a non-thalidomide treatment arm. Multiple testing was accounted for by label-swapping permutation. Pairwise epistasis was examined and recursive partitioning analysis was used to build
a multi-SNP model for thalidomide related VTE.
Left is a diagram showing a hypothetical model for thalidomide VTE consistent with association results from our study. On the
right, is decision tree derived from a recursive partitioning analysis of association results from thalidomide related analysis.
Thalidomide related neuropathy
Using the 3,400 custom array again, we undertook a case-control analysis of thalidomide related neuropathy. Treatment dosage, age, pathway, WHO status, toxicity stage and neuropathy
status overtime of the study were used to define thalidomide-related neuropathy status. Associations were reported if they were significantly associated with neuropathy following
permutation in both independent studies. We also reported vincrintine-related associations. A dutch-led analysis of bortezomib treated patients was also carried out utilizing the custom
array in Dutch, French and Czech patient samples.
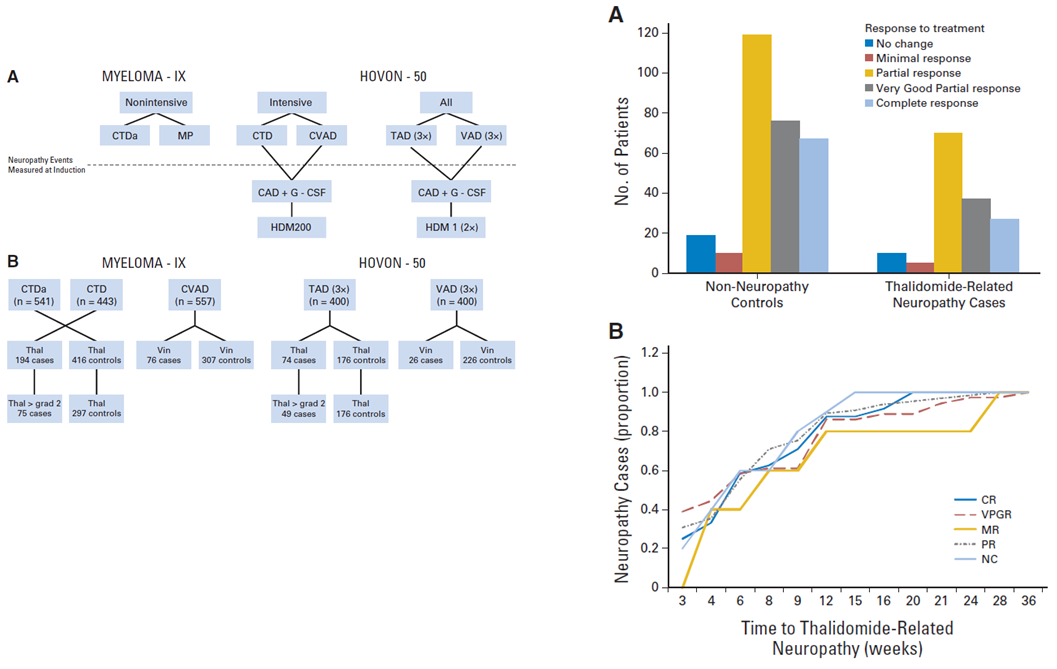
The left figure shows a breakdown of the treatment and study numbers. On the right, is a bar graph of disease response in myeloma IX cases, and
plot of time to thalidomide-related neuropathy by response.
Bone disease
An important serious adverse event in myeloma is bone disease that can be characterized with discrete or diffuse lytic lesion. I spent considerable time synchronizing bone disease status,
amongst the separate trials with the study. This was complicated by changes in patient cases report forms between the two UK trials. In addition, across the course of the My11 trial
there was a move to considering MRI in addition to X-ray bone scans. Finally, the majority of myeloma patients are older, so they are more susceptible to non-treatment related fractures.
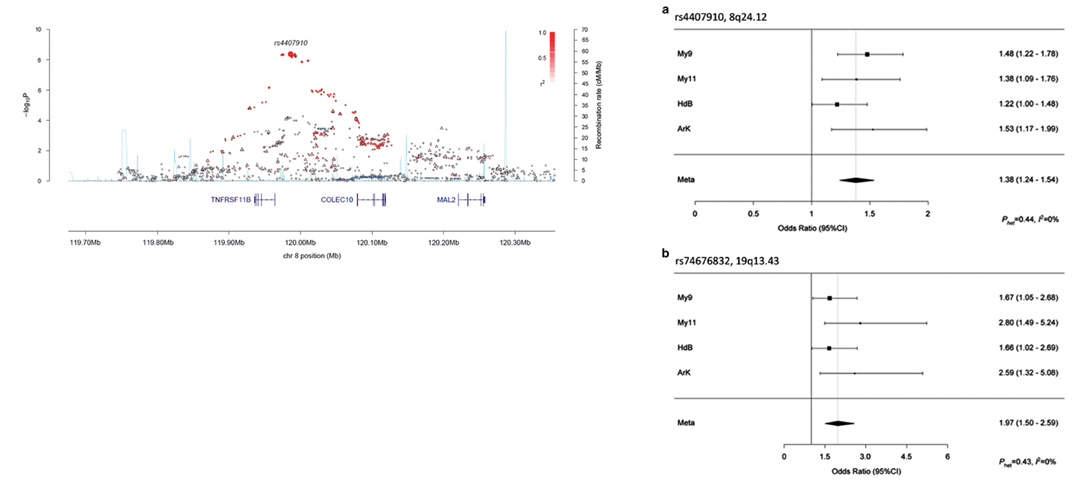
It was important to use all available demographics to define bone disease between cohort. We identified at genome-wide significance, P = 4.09 x 10-9 a previously described
variant in TNFRSF11B/OPG a rankL inhibitor. The variant was described in European non-myeloma bone disease study of 8557 patients at P = 7.6 x 10-10 published in
the Lancet (2008), they showed the top variant was a bone eQTL Twin study data. These results were important as they
suggested that underlying bone disease in myeloma was mechanistically similar to osteoporosis and that myeloma patients could potentially benefit from therapies targeting OPG/RANK/RANKL
pathways such as Denosumab.
On the left is the association plot of myeloma bone risk at TNFRSF11B/OPG, triangle points are from imputed SNPs. On the right is a forest plot of the two
strongest myeloma bone associations across the four nationwide Myeloma trials.
OS/PFS GWAS
I undertook extensive uni-variant analysis of known prognosis factors both clinical and genomic. Treatment, disease stage, population eigenvectors were used as in the German analysis as
covariates, as there were population differences across the German set, Heidelberg patients did better than there northern counterparts. Age was exclusively used as covariates
in the US analysis, as all German patients were young, and as UK treatment was already stratified for age. Although we had publishable results, this study design led to us measuring general
survival factors associated with outcome, rather tumour specific factors. I might suggest that single treatment studies may provide more fruitful results.
I also used ISS as a proxy to account for the more deleterious chromosomal lesions, which I believe was suboptimal.
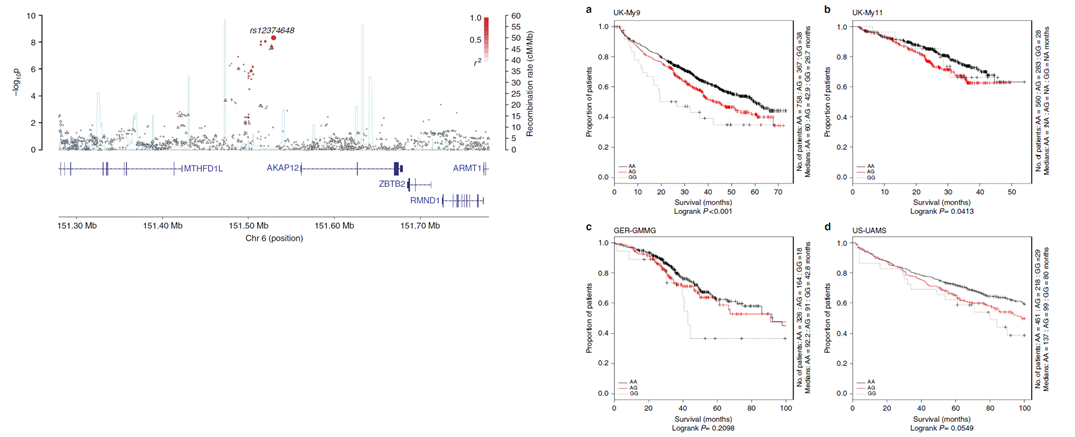
On the left, is an association plot of the meta-analysis results for overall survival across 6q25. On the right, Kaplan-Meier curves for each individual trial within the study for the sentinel SNP at 6q25.
Programs and packages
Association testing - PLINK versions 1.7-1.9, IMPUTE, EIGENSTRAT and SNPTEST; Meta-analysis - PLINK, META, METAL; Data-visualisation - ggplot2, SNAP, matplotlib, seaborn; HLA region analysis - SNP2HLA; eQTL and meQTL - matrixQTL, FastQTL and PEER.
References
◦ Genetic associations with thalidomide mediated venous thrombotic events in myeloma identified using targeted genotyping. Johnson DC, Corthals S, Ramos C, Hoering A, Cocks K, Dickens NJ, Haessler J, Goldschmidt H, Child JA, Bell SE, Jackson G, Baris D, Rajkumar SV, Davies FE, Durie BG, Crowley J, Sonneveld P, Van Ness B, Morgan GJ. Blood. 2008 Dec 15;112(13):4924-34. PMID: 18805967
◦ Genetic factors underlying the risk of thalidomide-related neuropathy in patients with multiple myeloma. Johnson DC, Corthals SL, Walker BA, Ross FM, Gregory WM, Dickens NJ, Lokhorst HM, Goldschmidt H, Davies FE, Durie BG, Van Ness B, Child JA, Sonneveld P, Morgan GJ. J Clin Oncol. 2011 Mar 1;29(7):797-804. PMID: 21245421
◦ Genetic factors underlying the risk of bortezomib induced peripheral neuropathy in multiple myeloma patients. Corthals SL, Kuiper R, Johnson DC, Sonneveld P, Hajek R, van der Holt B, Magrangeas F, Goldschmidt H, Morgan GJ, Avet-Loiseau H. Haematologica. 2011 Nov;96(11):1728-32. PMID: 21791469
Neutral tumor evolution in myeloma is associated with poor prognosis. Johnson DC, Lenive O, Mitchell J, Jackson G, Owen R, Drayson M, Cook G, Jones JR, Pawlyn C, Davies FE, Walker BA, Wardell C, Gregory WM, Cairns D, Morgan GJ, Houlston RS, Kaiser MF. Blood. 2017 Oct 5;130(14):1639-1643. PMID: 28827410.
◦ Genome-wide association study identifies variation at 6q25.1 associated with survival in multiple myeloma. Johnson DC, Weinhold N, Mitchell JS, Chen B, Kaiser M, Begum DB, Hillengass J, Bertsch U, Gregory WA, Cairns D, Jackson GH, Försti A, Nickel J, Hoffmann P, Nöethen MM, Stephens OW, Barlogie B, Davis FE, Hemminki K, Goldschmidt H, Houlston RS, Morgan GJ. Nat Commun. 2016 Jan 8;7:10290. PMID: 26743840
◦ Genetic factors influencing the risk of multiple myeloma bone disease. Johnson DC, Weinhold N, Mitchell J, Chen B, Stephens OW, Försti A, Nickel J, Kaiser M, Gregory WA, Cairns D, Jackson GH, Hoffmann P, Noethen MM, Hillengass J, Bertsch U, Barlogie B, Davis FE, Hemminki K, Goldschmidt H, Houlston RS, Morgan GJ. Leukemia. 2016 Apr;30(4):883-8PMID: PMID: 26669972